Insights
12 April 2018
A solution for improving recruitment into early Alzheimer’s disease clinical trials
Screening patients into early stage Alzheimer’s trials can be costly and time-consuming. Kenton Zavitz, PhD, Director of Clinical Affairs proposes a potential solution for improving recruitment into clinical trials.
Key points
- Screening patients into early Alzheimer’s trials can be costly and time-consuming.
- Screening the wrong patients into early Alzheimer’s trials may cause trial failure.
- CANTAB Recruit offers a solution with a highly sensitive cognitive assessment pre-screening tool that can be administered on a home computer to reduce the rate of costly in-clinic screen failures.
The Challenge
We have previously described the challenge of detecting early stage Alzheimer’s disease (AD), now we offer a potential solution.
As we know the cost of screening a single patient prior to determining suitability for trial enrolment may be many of thousands of dollars and the screen fail rate can easily exceed 70% of subjects screened, further increasing costs and timelines. Failure to recruit the planned number of participants in a timely manner can put a clinical development program at risk for clinical and commercial failure (Hughes 2012, Grill 2014). Indeed, 80% of delays to drug development programmes are attributed to unfulfilled enrolment numbers (PharmaFile 2016), and each day a drug release is delayed can lose the sponsor $8 million (Cutting Edge Information 2004). Considering all of these factors, finding the right patients at the right time is key to successful drug development.
Cambridge Cognition have developed a tool to help fill the gap in finding cognitively impaired subjects off-site for further screening on-site.
CANTAB Recruit: A technological solution for targeted recruitment into early Alzheimer’s disease trials
CANTAB Recruit is a computerized and automatically administered cognitive assessment tool for pre-screening participants into early AD clinical trials. The platform is web-based, which allows for potential participants to register and complete the assessment remotely without the need to attend a clinic. Results from the cognitive assessment, and participant demographic information are recorded, and used to determine a participant’s suitability for further in-clinic screening procedures.

Web-based testing with CANTAB Recruit
Paired Associates Learning (PAL): A sensitive cognitive screen for early Alzheimer’s disease
CANTAB Recruit employs the Paired Associates Learning (PAL) task which is a sensitive measure of visual episodic memory and learning and is associated with hippocampal and temporal-frontal network function (de Rover 2011, Nathan 2015).

The Paired Associates Learning (PAL) task
The PAL task has been selected for its reliability, validity and sensitivity to subtle episodic memory impairment that is correlated with brain beta-amyloid burden and cerebral spinal fluid (CSF) tau levels (Barnett 2011, Nathan 2015, Nathan 2017, Scheltens 2014, Zavitz 2017). Figure 1 visualises the relationship between CANTAB PAL performance and these CSF biomarkers.

Figure 1. Correlations between CANTAB PAL and cerebral spinal fluid (CSF) biomarkers of tau and beta-amyloid, converted to Cohen’s d estimates of effect size. Of the CSF biomarkers investigated, CANTAB PAL correlated best with beta-amyloid (Zavitz 2017).
Furthermore, lower hippocampal volume is associated with poorer episodic memory, as indexed by a higher number of PAL errors (Nathan 2015, 2017). This relationship is visualised in figure 2.
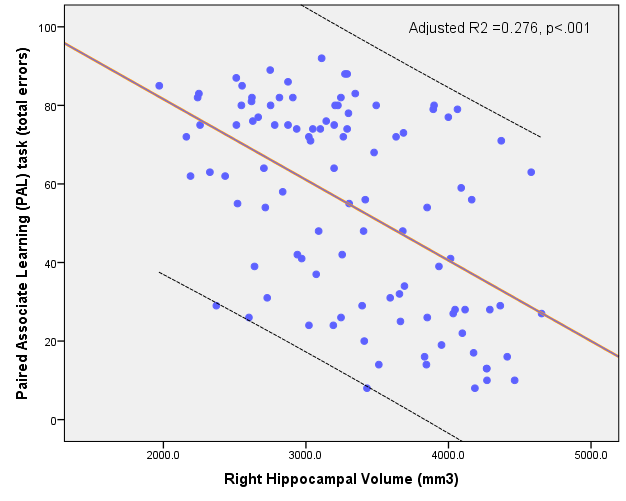
Figure 2. The association between hippocampal volume and PAL errors (Nathan 2015).
Subjects with mild cognitive impairment (MCI) who perform poorly on PAL at baseline are a stable group with a clinically-relevant impairment that worsens over time (Cormack 2015). This finding presents the CANTAB PAL task as a sensitive and specific indicator of amnestic MCI.
Together these observations have implications for identifying patients at risk of progressing towards Alzheimer’s dementia and enriching a more homogenous population for clinical trials with fronto-striatal and hippocampal dependent attention and memory deficits, neurodegeneration and CSF biomarker abnormalities consistent with prodromal AD populations (Nathan 2015, Nathan 2016).
CANTAB Recruit: Offsite screening to reduce onsite failures
According to a meta-analysis in persons without dementia (Jansen 2015), the prevalence of cerebral amyloid pathology in the general population over 50 years is 29%. The proportion is higher amongst people with MCI (51%) than in those with normal cognition and increases further still with age (Jansen 2015).
Administration of the PAL task using CANTAB Recruit can help identify individuals who are likely to be amyloid positive thereby reducing the number of subjects required for in-clinic screening to reach the target enrolment number.
Results from subjects aged 50+ enrolled in the EDAR study¹, indicate that sample ‘enrichment’ is based on the sensitivity and specificity of the PAL task together with the pre-test probability of amyloid positivity in the general population (i.e. 29%).
As the level of impairment on the PAL tasks increases, the number of subjects that require CSF sampling (or amyloid PET imaging) to identify cerebral amyloid positivity is reduced (as shown in figure 3).
The percentage reduction is a trade-off between sensitivity and specificity (maximizing true positives and minimizing false negatives) with the maximum enrichment in the example below, falling at PAL error cut point E.
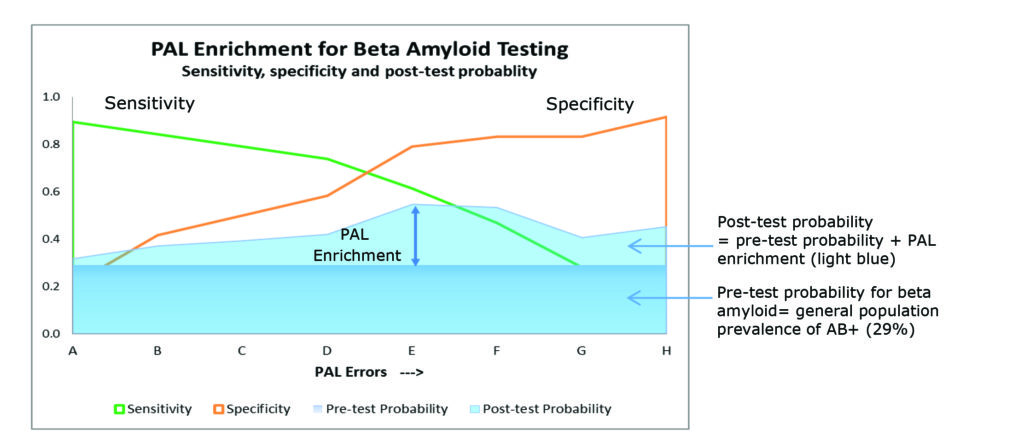
Figure 3. Sensitivity and specificity of PAL for enriching beta-amyloid testing. ¹ Based on data from subjects aged 50 plus from the European EDAR study (Early Diagnosis of AD and as Marker for Treatment Response).
CANTAB Recruit can be tailored to the sponsor’s specific trial requirements, such as for more impaired (MCI) versus less impaired (‘asymptomatic at risk for AD’) populations.
CANTAB cognitive testing: The same reliable results in-person and online
In-person assessments present a massive time-burden for participants, and resources-burden for the study team.
At CTAD 2017, our Director of Research & Innovation, Dr Francesca Cormack showed that web-based cognitive assessments offer the same high-quality results as in-person assessments. This finding presents a huge step forward for screening patients into clinical trials. Figure 4 illustrates the comparability between the two situations (home, online) with performance on CANTAB PAL.

Figure 4. The distribution of scores on PAL total errors adjusted (TEA) and first trial memory score (FTMS) outcome measures, reaction time and reaction time variability from in-person and web-based testing. Mean scores were equivalent in the two conditions (Cormack 2017).
CANTAB Technology: Acceptable and feasible for older adults
So we have the technological capability to perform remote, computerised assessments with adults at-risk of AD: but is this method of delivery acceptable and feasible for the adults themselves?
A large-scale survey of older adults (55-96 years old) living in Australia, found a preference for computerised cognitive assessments (54.3%) (figure 5), and home-based, unaccompanied testing (73.8%) (Earl 2017).
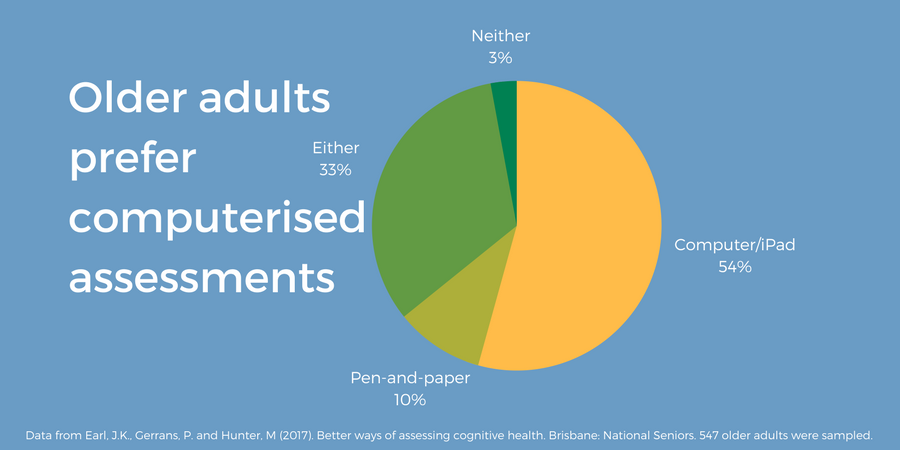
Figure 5. 547 older adults were asked “what type of assessment would you prefer?” Data from Earl et al. (2017). Graphic produced by Cambridge Cognition.
Next steps
Here we have presented CANTAB Recruit as a potential solution for targeted, cost-effective recruitment into early AD trials. Watch our webinar for a full discussion of the issues, and potential solutions, for successful recruitment into early AD treatment trials.
To find out more about our tools to help recruitment rates for your study, speak to one of our specialists about using data to enrich study populations.
This article was written by Dr Kenton Zavitz, with assistance from Dr Rosie Abbott and Dr Sally Jennings
References
Barnett JH, Blackwell AD, Damian M. Cognitive & CSF Biomarkers in Older Adults, MCI, and Dementia. ICAD 2011 presentation.
Cormack F, Backx R, Cotter J, Taptiklis N, de Cock L, Zavitz K, Barnett J. A comparison of in-person and web-based computerised cognitive testing using CANTAB. CTAD 2017 presentation.
Cormack F, Barnett JH, Nathan PJ et al. Stability of amnestic MCI: CANTAB paired associate learning as a predictor of a consistent diagnosis. AAIC 2015 presentation.
Cutting Edge Information. Accelerating Clinical Trials: Budgets, Patient Recruitment and Productivity. 2004
de Rover M, Pironti VA, McCabe JA, Acosta-Cabronero J, Arana FS, Morein-Zamir S, … Sahakian BJ. Hippocampal dysfunction in patients with mild cognitive impairment: a functional neuroimaging study of a visuospatial paired associates learning task. Neuropsychologia 2011, 49(7), 2060–70.
PharmaFile (2016). Clinical trials and their patients: The rising costs and how to stem the loss.
Grill JD, Galvin JE. Facilitating Alzheimer disease research recruitment. Alzheimer Dis Assoc Disord. 2014 Jan-Mar;28(1):1-8.
Hughes L, Kalali A, Vanbelle C, Cascade E. Innovative Digital Patient Recruitment Strategies in Prodromal AD trials. Poster at CTAD Annual Meeting, October 29-31, 2012.
Jansen WJ, Ossenkoppele R, Knol DL et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015 May 19;313(19):1924-38.
Nathan PJ, Abbott R, Galluzzi S et al. Characterization of cognitive function with the CANTAB in individuals with amnestic MCI in relation to hippocampal volume, amyloid and tau status: Preliminary baseline results from the PharmaCog/European-ADNI Study. AAIC 2015 presentation.
Nathan PJ, Abbott R, Lim YY, et al. CSF b-Amyloid and APOE ɛ4 Related Decline in Episodic Memory over 12 months measured using the CANTAB in individuals with amnestic Mild Cognitive Impairment (MCI): Results from the European-ADNI/Pharmacog study. AAIC 2016 presentation.
Nathan PJ, Ying Lim Y, Abbott R, Galluzzi S, Marizzoni M, Babiloni C, … Pi Sunyer A. Association between CSF biomarkers, hippocampal volume and cognitive function in patients with amnestic mild cognitive impairment (MCI) , on behalf of the PharmaCog Consortium. 2017 https://doi.org/10.1016/j.neurobiolaging.2017.01.013
Scheltens P et al. Baseline patient characteristics from the Phase 3 SCarlet RoAD trial, a study of gantenerumab in patients with prodromal AD. Presented at CTAD 2014, JPAD 2014; 1:231-2
Zavitz K, Abbot R, Cormack F, Dente P, Barnett J. Enriching participant eligibility for clinical trials through pre-screening for cognitive deficit. CTAD 2017 presentation.
You may also be interested in:
Author:


